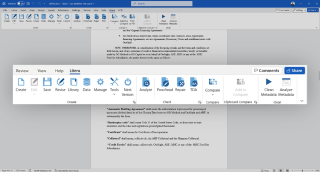
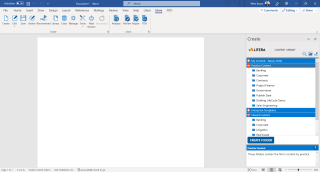
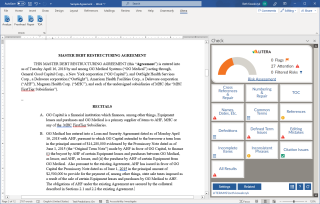
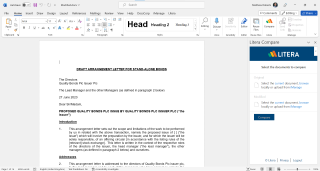
All Your Drafting Tools in One Ribbon
Legal professionals switch apps 1,200 times daily, wasting four hours a week. Litera Draft automates document drafting for lawyers in MS Word, saving valuable time.

Empowering Legal Professionals
Remove the complexity of legal document drafting and easily create, check, compare, clean, and publish impeccable documents without switching applications.
Focus On High-Value Work without Multiple Point Solutions or Manual Drafting
Avoid Workflow Interruptions with One Simple Drafting Suite
Mitigate Risks That Damage Reputation and Client Confidence
Benefits of Litera Draft
Create Documents 85% Faster
Efficiently create high-quality first drafts with enterprise-approved templates and a content library.
Proofread, Style, and Repair with Ease
Enable legal professionals to deliver high-quality documents with AI-powered proofreading, styling, and document repair.
Collaborate Securely to Compare, Clean, and Share Documents
Take document collaboration to the next level by easily comparing document versions in seconds, cleaning risky metadata, and sharing encrypted documents.
Securely Publish to PDF
Simplify PDF workflows and ensure legal professionals can seamlessly edit, redact, bind, and publish documents in less time.
An IT Game-Changer
Forget about managing multiple drafting points solutions. Consolidate your drafting technology into one suite with Litera Draft.
Avoid Add-In Conflicts with a Drafting Suite That Sits in One MS Word Ribbon
Eliminate Disparate Point Solutions and Consolidate Your Drafting Tools to Improve Adoption
Work with One Vendor That Meets Your Needs through High-Quality Support
Powered by Litera AI+: Generative AI You Can Trust
Enjoy secure and scalable GenAI capabilities across Litera tools.
Litera AI+ is integrated with Microsoft Azure’s OpenAI services to ensure your data remains safe and secure. We’re committed to responsibly innovating with GenAI to solve your top challenges.
Future-Ready Legal Practices eBook
Are you ready to experience the future of drafting?
Partner with Litera and experience the innovation.
Resources
Resources for Litera Draft
Learn more about how Litera Draft can help your law firm streamline document drafting.